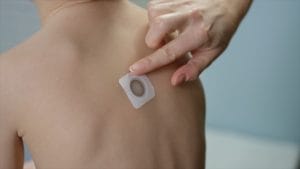
The FDA says it won’t approve DBV Technologies’ Viaskin Peanut, the novel skin patch allergy desensitization treatment, at least not in its current form.
In announcing the drug regulator’s decision, DBV said in a press release that the FDA raised concerns about Viaskin patch’s adhesion to the skin and the impact of that on treatment. To address the concerns, DBV says the FDA calls for patch modifications, followed by “a new human factors study.”
The company’s leadership vows to comply with the FDA’s requests and move forward. “We are very disappointed in the FDA’s response, but continue to believe in the potential of Viaskin Peanut,” Daniel Tassé, CEO of DBV, said in the August 4 release.
DBV says the FDA also requested further clinical data on chemistry and manufacturing. No safety concerns were raised related to the therapy.
Allergic Living asked DBV whether the human factors study would mean a full new clinical trial. Tassé replied: “We plan to request a meeting with the agency in the coming days to further understand the feedback and determine next steps.” He said updates would be provided after that meeting.
The Viaskin Peanut patch was designed to treat peanut-allergic children aged 4 to 11 through a skin treatment known as epicutaneous immunotherapy (or EPIT). The patch, which contains 250 micrograms of peanut protein, is applied daily.
As for families whose children who have taken part in the Viaskin Peanut trial extension research and have been applying the patches, Tassé recommends they speak to their trial investigators. “All our studies are continuing as planned, and the current patch remains available to patients enrolled in studies,” he told Allergic Living.
“DBV remains very committed to peanut allergy treatment and will meet with FDA to discuss [the FDA letter] and look at modifying the patch,” he said. “We recognize the burden that peanut allergies can place on patients living with peanut allergies and their families, and remain dedicated in our mission to develop innovative treatments for patients with food allergies.”
In the press release, DBV said the EPIT patch technology platform “lends itself well to potential modifications to enhance patch functionality.”
Buffer Against Reactions
The patch therapy’s goal is desensitization, or increasing an allergic child’s threshold for consuming peanut before experiencing symptoms of an allergic reaction. This affords a safety buffer to protect against serious reactions, in case of accidental peanut exposures.
In mid-March 2020, the FDA asked DBV questions about patch adhesion and in late June, DBV said it had supplied answers from peer-reviewed data. As it awaited word on its biologics license application, the French biopharmaceutical company also announced it would begin restructuring. This is being done to preserve cash flow, and includes job layoffs.
The nonprofit FARE (Food Allergy Research and Education) expressed disappointment with the FDA’s decision to not approve the current Viaskin therapy, but also held out hope for a revised Viaskin design.
“We are confident that the concerns raised by the FDA can be addressed and that this development will lead to an improved version of the patch,” said FARE CEO Lisa Gable. She expressed optimism that this would “ultimately provide more treatment options for not just peanut-allergic children, but for all 32 million Americans living with food allergies.”
Viaskin’s Ups and Downs
Back in 2012, with growing interest in food allergy treatments, the FDA gave the Viaskin Peanut patch therapy fast-track status to receive priority review. However, in October 2017, the patch hit a hurdle in clinical development when its Phase 3 study missed a key statistical target set out by the agency.
Yet, the results did show promise – with 35.3% of patients showing significant response to the peanut patch after one year. Previous and later studies would show young patients had better success when they were on the patch for three years.
In fact, the results of an extension study, published in January 2020, showed that 52 percent of peanut-allergic kids on the Viaskin patch could consume up to 1,000 milligrams of peanut protein before starting to react. That’s about 3 to 4 peanuts.
The peanut patch treatment also hit an earlier roadblock with the FDA. In December 2018, DBV voluntarily withdrew its license application with the FDA after the agency said the application to lacked “sufficient detail regarding data on manufacturing procedures and quality controls.” The company resubmitted in August 2019, saying it had addressed the issues.
While DBV had hoped to be the second approved peanut allergy treatment in the United States in 2020, it will now have a long road ahead to FDA license reconsideration. In January 2020, the FDA gave the green light to Aimmune Therapeutics’ Palforzia oral immunotherapy, making it the first-ever approved food allergy treatment.
Related Reading
DBV’s News Release on FDA’s Response Letter
3 in 4 Kids See Benefit on Patch Therapy after 3 Years





