Desensitizing therapies are emerging for food allergies, and being considered for approval by the FDA. But some in the medical community raise this question: Is desensitization, which is not a cure, enough to improve a food-allergic person’s quality of life?
A new study of families involved in DBV Technologies’ Viaskin Peanut patch therapy clinical trials gives insights into an answer. It found significant overall improvement in quality of life related to gaining greater peanut tolerance on the patch treatment. As well, questionnaires filled out by both children and parents showed quality of life gains in specific areas.
With parents, “the areas where we saw the most impact were the emotional impact, food-related anxiety, and social and dietary limitations,” Dr. Todd Green, DBV’s vice president of medical affairs for North America, told Allergic Living. In the children, aged 8 to 12, “when they were asked questions around avoidance or accidental exposure, that’s where that’s where they reported the most improvement,” he said.
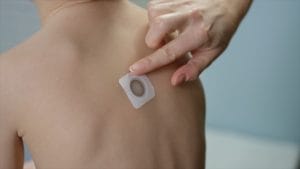
Children in the Viaskin Peanut studies were slowly exposed to their allergen through the patch that releases peanut proteins through the skin and stimulates the immune system. The goal is desensitization, which means a child must still practice peanut avoidance, but it will take a considerably higher protein exposure to cause the treated patient to react.
The quality of life analysis was led by Audrey Dunn Galvin, PhD, a lecturer at the School of Applied Psychology at Ireland’s University College Cork. Dunn Galvin, who has developed the state-of-the-art standardized questionnaire for food allergy quality of life research, presented her findings at the November 2019 ACAAI allergists’ meeting. She said the questionnaire data showed treatment with the Viaskin patch “resulted in significant improvement in quality of life.”
Parents, Kids Surveyed Separately
In the Viaskin Peanut Phase 3 study called Pepites, children were placed in either an active treatment group, and wore a 250-microgram of peanut protein patch daily, or they were in the control arm, and wore a placebo patch. (They didn’t know which group they were in.) At the end of 12 months, all participants, including those on the placebo, received Viaskin treatment for an additional year in a follow-up study.
Green says patients and their families completed the quality of life questionnaires upon entry to the study, then at 12 months and 24 months. Surveys were filled out by 209 parents and 105 children in the active treatment group, and 96 parents and 47 children in the placebo group.
The impact of quality of life improvements was greatest among families where a child had met the study’s primary endpoint of being able to consume 300 milligrams or more of peanut. By 24 months, Green said families gained a greater sense of whether the amount it took to cause a peanut reaction had substantially increased.
Haven’t Known Before
“The studies indicate this potential therapy raises your eliciting dose,” he said. “This hopefully then translates to a reduced risk of reaction and hopefully also improves your quality of life.”
Green says of the questionnaire results: “These are some of the most exciting data we’ve seen. We haven’t known that until now.”
He notes that on the patch, the 24-month mark is important. This is where families begin to know whether the eliciting (or threshold) dose before reacting to the allergen is moving in the right direction. While the Viaskin patch was initially intended to be worn for 36 months, it’s now been extended for up to five years for patients.
Green says that timeframe isn’t surprising. “With injection immunotherapy, if someone’s doing well with their allergy shots for cat or dust mite, that typically takes three to five years at maintenance. This is a concept that’s familiar to allergists.”
Earlier this year, Aimmune Therapeutics also reported positive quality of life data from its extension study of the Palforzia (or AR101) peanut oral immunotherapy treatment. Paflorzia is the other leading food allergy therapy being considered for FDA approval.
Related Reading:
ACAAI 2019 Report: OAS in Kids, Penicillin Allergy Label, Twitter Impact