 Benjamin Sun
Benjamin Sun But in the span of one year in a clinical trial for a new biologic drug called dupilumab, “my eczema has totally cleared up. Well, 99 percent of it has,” the 13-year-old told Allergic Living.
The tween is amazed to be able to control eczema that “was really painful. It ruined my daily routine.” At school, other students would sometimes treat him as if he was contagious. “People would just cringe away and not talk to me,” he recalls.
Since starting dupilumab in 2018, Tracy Wang, Benjamin’s mother, says his quality of life has drastically improved. The treatment, taken by injection twice a month, first at the doctor’s office, then continuing at home, has been developed for individuals with moderate-to-severe eczema that’s not controlled by topical steroids. In 2017, the biologic was approved for treatment in such adults across North America.
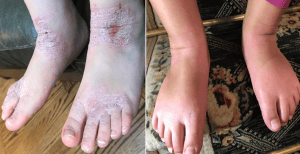 A patient’s skin shown before and after dupilumab treatment.
A patient’s skin shown before and after dupilumab treatment. “It is very exciting to have this treatment available for adolescents,” says Dr. Emma Guttman-Yassky, the dermatology professor at the Icahn School of Medicine at Mount Sinai who has led the research into dupilumab.
Dupilumab is a monoclonal antibody, which acts like a homing device to specifically target two chemical messengers – interleukin-4 and interleukin-13, which are known promoters of allergic disease. By blocking the two interleukins, dupilumab injections are halting the underlying inflammation of atopic dermatitis.
 Dr. Emma Guttman-Yassky
Dr. Emma Guttman-Yassky Guttman-Yassky says studies with the biologic are also underway for children under the age of 12 with moderate-to-severe eczema. “There is a pediatric plan in place and I think that is very important.” And not just for eczema itself. She notes that in younger children, there is a narrow window of opportunity to intervene in the so-called “allergic march” – before they potentially go on to develop related diseases such asthma or food allergy.
For Benjamin, the therapy has been nothing short of life-changing. He doesn’t itch at night anymore. He sleeps regularly and doesn’t miss class or sports activities. He says instead of large red patches, he sometimes has “just tiny bumps” on his skin. His hair has thankfully also grown back – after he had lost all of it to alopecia (dupilumab is also being studied to see how it helps with that condition).
“I can go back to a normal routine,” he says.
Related:
Breakthrough Therapy Changing the Lives of Those With Eczema
Hope for Eczema Drug Ranges from Asthma to Food Allergy
Get more news from Allergic Living to your inbox.





