Do you own EpiPens that are part of the March 31, 2017 recall? Pharmaceutical company Mylan provides the following step-by-step guidance on how to replace these EpiPens and EpiPen Jrs.
As well, Allergic Living gets answers from Mylan on some key questions about the devices that were recalled.
Update provided by Mylan on the recalled lots of EpiPen
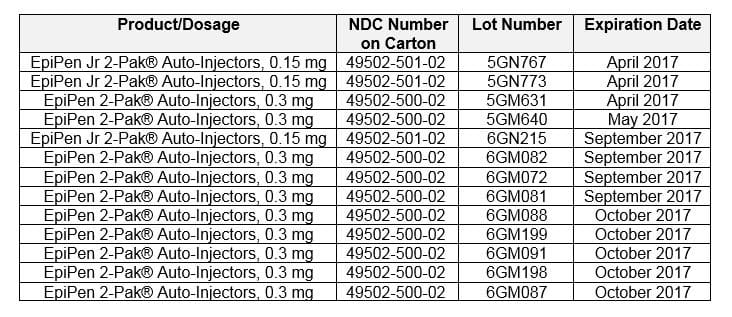
STEP 1: Check the lot number on your carton or device to see if your EpiPen Auto-Injector is affected by the recall.
Recalled Lots in U.S.
STEP 2: If your EpiPen Auto-Injector has been recalled, contact Stericycle at 877-650-3494 to obtain a voucher code for your free replacement product. Stericycle also will provide you with a pre-paid return package to ship the product back to Stericycle.
STEP 3: Visit your pharmacy with your voucher information to redeem your free replacement.
STEP 4: Send your recalled product to Stericycle. Do not return any devices affected by the recall until you have your replacement in hand. Stericycle’s hours of operation are Monday-Friday 8 a.m. to 10 p.m. ET, and Saturday and Sunday 8 a.m. to 5 p.m. ET.
Mylan’s note on wait times: “We are aware that callers have experienced extended wait times and sincerely apologize for the inconvenience that this has caused. We are working closely with Stericycle, our recall management vendor, to increase staff trained to manage calls more quickly and reduce call wait times. We appreciate your patience.”
Answers from Mylan to Questions About the Recall
Q. How many device failures have there been, and were any of them in North America?
Mylan’s spokesperson: “The recall is being conducted as a result of the receipt of two previously disclosed reports, one from Europe and one from Asia Pacific, of failure to activate the device due to a potential defect in a supplier component.”
Q. What happened to the patients involved since these two devices didn’t work?
Mylan’s spokesperson: “In both situations, patients were able to obtain treatment through the use of an alternate EpiPen Auto-Injector. Both reports are related to the single lot that was previously recalled [this was prior to the U.S. recall].”
Q. Other EpiPens lots, including the new generic auto-injector, are not part of the voluntary recall. Can you explain why manufacturer Meridian is only targeting some U.S. devices?
Mylan’s spokesperson: “Testing and analysis across the potentially impacted lots has not identified any units with a defect. However, the recall is being expanded by Meridian to include additional lots as a precautionary measure out of an abundance of caution.”
Q. Still, it’s not obvious why some EpiPens might have the potential component flaw, but not others. Why this difference?
Mylan’s spokesperson: “The recall is being expanded to include additional lots as a precautionary measure due to the commonality of a supplier component lot in these lots of EpiPen.
To reinforce, testing and analysis across the potentially impacted lots has not identified any units with a defect. The recall is a precautionary measure.”
See also: Original article – EpiPen Recall Expands to U.S. and Canada.