A new drug to treat moderate-to-severe eczema has been approved by the U.S. Food and Drug Administration, signaling a potential breakthrough for adults with the chronic skin condition.
Dupilumab, which has the brand name Dupixent, is an under-the-skin injection. It’s the first biologic medicine for atopic dermatitis, commonly called eczema. The treatment contains living cells that act to block two proteins, IL-4 and IL-13, which are key to eczema’s inflammation.
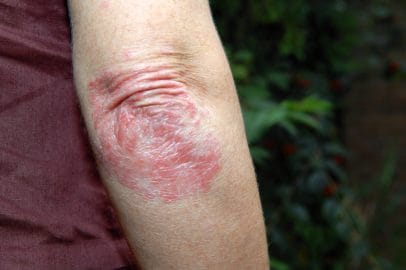
People with moderate-to-severe eczema suffer from flare-ups of painful, cracked, dry and broken skin. This drug would treat the root of the disease rather than its symptoms.
“People with moderate-to-severe atopic dermatitis cope with intense, sometimes unbearable symptoms that can impact them for most of their lives,” Julie Block, president and CEO of the National Eczema Association, said in a press release. She noted that there have been few options available to treat people with this level of atopic dermatitis who have uncontrolled disease.
“That’s why today’s approval of Dupixent is so important for our community,” said Block. “Now we have a treatment that is expected to help address patients suffering from this devastating disease.”
In three placebo-controlled clinical trials, more than 2,000 participants showed clear or almost clear skin. After 16 weeks of treatment, the participants also experienced a significant reduction in itch. Dupixent can be administered at home every other week after an initial dose with a doctor.
In terms of side effects, some people can have serious allergic reactions to dupilumab. Also in the trials, some experienced eye problems, including conjunctivitis and inflammation of the cornea.
Like most biologics, Dupixent is expensive with a list price (or wholesale acquisition cost) of $37,000 a year. Sanofi and Regeneron, the manufacturers of the medicine, say the actual cost to patients and insurers will be lower. (For more information, call 1-844-387-4936 or visit www.Dupixent.com.)
In Canada, the Health Canada agency is also reviewing possible approval of this therapy.
Dupilumab is now being studied in children (6 months to 11 years) with severe atopic dermatitis and teens aged 12 to 17 with moderate-to-severe eczema.
Related:
Breakthrough Therapy Changes Lives of Those with Eczema
Report on study: Novel Eczema Drug Giving Patients New Lease On Life