April 25 Update: Following a safety review, the FDA and the CDC have lifted the pause in the use of the Johnson & Johnson coronavirus vaccine in the U.S. That pause came into effect on April 13, after reports of six U.S. cases of blood clots and low levels of blood platelets in women between the ages of 18 and 48. The FDA now says data show the risk of this blood clot syndrome “is very low.” The agencies stress that adverse events are rare (6.8 million doses of the J&J vaccine had been given at the time of the pause). See also our FAQ on the Pfizer and Moderna mRNA vaccines here.
The U.S. Food and Drug Administration approved emergency use of Johnson & Johnson’s coronavirus vaccine in late February. With the Centers for Disease Control and Prevention adding its endorsement of the vaccine’s safety and effectiveness on February 28, a third coronavirus vaccine has begun rolling out across the U.S.
An FDA analysis determined J&J’s one-shot vaccine provides strong protection against severe COVID-19 illness. Considered from the allergy perspective, there were no reports of anaphylaxis among nearly 22,000 adults who received the vaccine in a Phase 3 clinical trial.
“The J&J vaccine appears to be safe for the overwhelming majority of people with food or environmental allergies,” commented Dr. Scott Commins, an allergist and associate professor of medicine at University of North Carolina at Chapel Hill.
The FDA granted the vaccine emergency use status a day after an independent advisory panel of experts voted unanimously in favor of the J&J shot. With the sign-off of CDC director Dr. Rochelle Walensky, the company says an initial 3.9 million doses of the vaccine are starting to roll out. [On March 5, Canada also approved emergency use of the vaccine.]
In a Phase 3 clinical trial, the J&J vaccine was 66 percent effective in preventing moderate to severe COVID illness in adults 18 and older across multiple countries. In U.S. study participants specifically, it showed 72 percent effectiveness. Crucially, the vaccine was 85 percent effective in preventing severe or critical illness.
Vaccine’s Level of Protection
There were no hospital admissions or deaths among trial participants receiving the vaccine (as opposed to a placebo) after 28 days after vaccination. While not as protective as two doses of the Pfizer-BioNTech or Moderna mRNA vaccines, which have shown efficacy of about 95 percent, the J&J vaccine only requires a single dose.
“People may be concerned that if they get the J&J vaccine they still have a chance of being infected with COVID-19, but the important thing is if they get infected they don’t get seriously ill and risk dying,” says Dr. James Baker, a University of Michigan allergist-immunologist. “This vaccine provides value as another option, especially now when vaccines are limited supply.”
Another advantage of the J&J vaccine is ease of transport and storage. It can be safely refrigerated for several months, unlike the mRNA vaccines, which must be kept frozen and used quickly after thawing.
With the J&J vaccine, protection against coronavirus was measured at 14 days after the vaccine, and at 28 days. After 28 days, there were no COVID-19 hospital admissions or deaths in the vaccinated group compared with 16 hospitalizations and seven deaths in the placebo group.
J&J’s Safety Profile
The most common side effects to the J&J vaccine were injection site pain, headache and fatigue, which are commonly seen after many types of vaccines. There were no reports of anaphylaxis during the Phase 3 trial, which have been reported in a small number of cases following injection with the Pfizer and Moderna vaccines.
Severe reaction following an mRNA coronavirus shot is considered rare. The rate of anaphylaxis is 4.7 cases per one million doses of the Pfizer vaccine and 2.5 cases per one million doses of the Moderna vaccine, according to a CDC expert’s study of the first 17.5 millions shots given in the U.S.
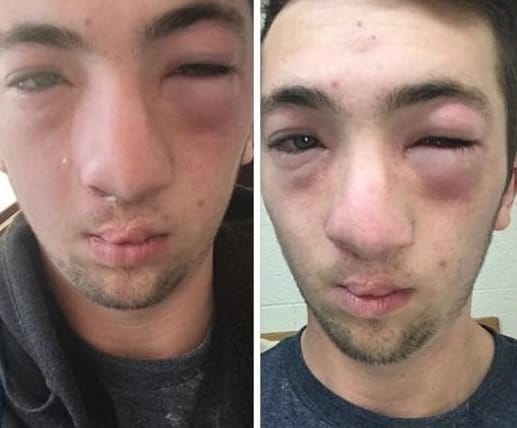
Among the 44,000 in the J&J vaccine trial, 0.4 percent of those who received the vaccine and 0.3 percent of those who received the placebo reported symptoms such a rash or hives. There were two cases considered serious “hypersensitivity” – but not anaphylactic – reactions. This includes one participant who had hives starting two days after getting the shot and another with lip swelling four days later.
During the Feb. 26 advisory panel meeting, Dr. Macaya Douoguih, head of clinical development and medical affairs at Janssen, J&J’s vaccines division, said the company had just been informed that there are two preliminary reports of severe allergic reactions. This includes one case of anaphylaxis, which occurred during an ongoing South African trial.
Baker, director of the Mary H. Weiser Food Allergy Center at University of Michigan, says a single case of anaphylaxis would not be a cause for concern.
Vaccine Ingredients
As for ingredients, the J&J vaccine contains no animal-derived proteins, including egg, gelatin or cow’s milk-derived protein, Commins says. Nor are there any animal-derived proteins used in the materials used to produce the vaccine, he adds, which should reassure people with the tick-related allergy known as alpha-gal syndrome.
The J&J vaccine does include polysorbate 80. Along with polyethelyne glycol (PEG), an ingredient in the Pfizer and Moderna vaccines, polysorbate has been associated with very rare allergic reactions.
But because polysorbate is so common – it’s found in many foods, cosmetics and other types of vaccines, including some flu shot – “people with sensitivity to polysorbate may already know,” says Commins.
In addition, Baker questions whether polysorbate is indeed a potential allergen. There are documented, if rare, cases of allergy to PEG, considered related to polysorbate. However, Baker says he and other allergists are not convinced that claims of polysorbate reactions are verifiable. “There is nothing in the [J&J] vaccine that we would consider a known allergen,” he says.
Because of PEG’s presence in the mRNA vaccines, Baker’s clinic has been inundated with calls from worried patients requesting PEG allergy skin tests. Of over 30 tests done so far, all were negative, he says.
How the Vaccines Work
The Pfizer-BioNTech and Moderna vaccines both use messenger RNA (mRNA), a bit of synthetic genetic material that prompts cells to make the COVID-19 “spike” protein, which the virus uses to infect healthy cells. The production of the spike protein triggers immune system to produce antibodies and other immune cells that can mobilize quickly to fight the virus.
The J&J vaccine, in contrast, uses a modified, non-infectious form of a human adenovirus (which can’t replicate) to deliver genetic instructions to make the spike protein. The body recognizes the spike protein as an invader and produces antibodies against it. If the antibodies encounter the actual virus at a later date, the immune system is primed to destroy the virus before it causes illness.
The efficacy of the vaccine varied somewhat according to geography. The vaccine was 72 percent effective at preventing moderate to severe COVID-19 in the U.S., compared with 61 percent in Latin America and 64 percent in South Africa. The J&J vaccine also appears to reduce transmission of the virus to others by 74 percent compared to a placebo.
The CDC advises that any facility administering COVID-19 vaccines, including the newly authorized J&J vaccine, should have trained medical personnel, supplies and equipment to manage anaphylaxis. Those with a history of severe allergic reactions to any cause should be observed for at least 30 minutes after vaccination. All others are to be monitored for at least 15 minutes.
Related Resources:
Few Severe Reactions to COVID-19 Vaccines, But Women Most Affected
FAQ on Pfizer and Moderna Vaccines & Allergies
Allergists’ Video: COVID-19 Vaccines and Reaction Risks