The FDA has issued an important alert to individuals with allergies and health-care professionals that some lots of Amneal and Impax Laboratories epinephrine auto-injectors (0.3 mg size) may be missing the yellow “stop collar.”
Impax Laboratories warns that, “if the auto-injector is missing a yellow ‘stop collar’ component, it has the potential safety risk of delivering a double dose of the product to a consumer.” This could lead to a serious overdose.
If this is the type of auto-injector you or a family member carry, see instructions below on how to visually inspect to see if the yellow collar is in place.
Impax Laboratories, is a subsidiary of Amneal Pharmaceuticals LLC, and distributes the Epinephrine Auto-Injector, USP in 0.3 mg and .15 mg sizes. Only the 0.3 size auto-injector is affected by this alert, and the auto-injectors have expiration dates between June 2020 and May 2021. (For specific lot numbers, see list in this letter.)
If the yellow stop collar is in place, the FDA advises that the device is safe to use. Impax Laboratories is asking pharmacists and health-care professionals to make all consumers who have purchased its company’s auto-injectors aware of the need to check their devices.
Following is Impax Laboratories letter to consumers, with instructions on how to visually inspect your devices to check for the yellow stop collar.
Impax Production Safety Advisory
Dear Consumer:
Impax Laboratories, LLC, a wholly owned subsidiary of Amneal Pharmaceuticals LLC, is providing important safety information concerning its Epinephrine Injection, USP Auto-Injector 0.3 mg. Impax is writing to inform you that some Epinephrine Injection, USP Auto-Injector 0.3 mg devices may not contain the yellow “stop collar” component (see pictures below). The yellow “stop collar” is one of several components that work together to assure proper dosing of the auto-injector.
If the auto-injector is missing a yellow “stop collar” component, it has the potential safety risk of delivering a double dose of the product to a consumer. An overdose of epinephrine has the potential to cause severe patient harm or death.
If you have received Amneal or Impax’s Epinephrine Injection, USP Auto-Injector 0.3 mg after December 20, 2018, Impax is requesting that you immediately perform a visual inspection as described below to confirm the presence of the yellow “stop collar”:
• Carefully inspect for the presence of the yellow “stop collar.”
• If yellow “stop collar” is present, then the product is safe to use. No further action is necessary.
• If the yellow “stop collar” is missing, call or e-mail Amneal Drug Safety Department using the contact information below, for instructions for the return and replacement of the auto-injector.
Amneal Pharmaceuticals Drug Safety Department: Phone: 1 (877) 835-5472; Email: [email protected]; Mail: Amneal Pharmaceuticals, 50 Horseblock Rd., Brookhaven, New York 11719
Visual Inspection Instructions
Note: If you have any questions about the steps below, or are unsure if the yellow “stop collar” is missing, please call Amneal at 1(877) 835-5472.
Step 1: Remove the auto-injector from the carrying case
Step 2: Place the auto-injector on a flat surface as shown below

Step 3: Locate the edge of the label that states “Peel here for further instructions.” Lift the label edge until you see the clear part of the auto-injector.

Step 4: Look for the YELLOW “stop collar”. See arrow below.
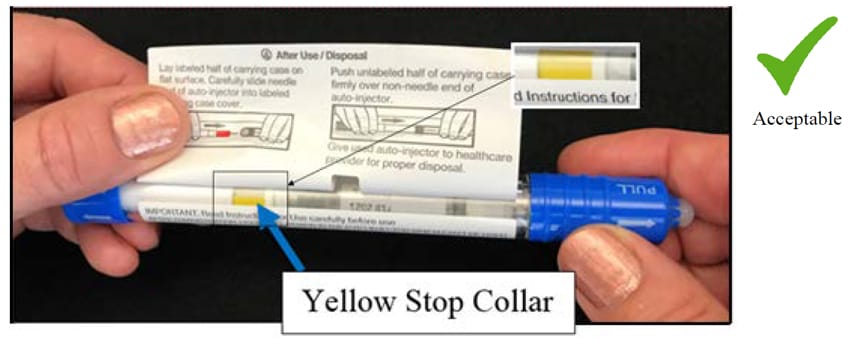

Please Note: If the yellow “stop collar” is missing, hold the unit (as shown below) and gently rotate the blue sheath remover and observe until the yellow “stop collar” comes into view. DO NOT pull or remove the blue sheath remover.

Important
• If yellow “stop collar” is present, then the product is safe to use. No further action is necessary.
• If the yellow “stop collar” is missing, contact Amneal Drug Safety Department [contact information is above], to make arrangements for the return of the auto-injector and a replacement at no additional cost.
Step 5: Re-wrap the label to its original position and place the auto-injector into the carrying case.
View final paragraphs of Impax / Amneal letter here.
The FDA alert notes: Patients should contact their pharmacy regarding a replacement epinephrine auto-injector before returning the defective device to Amneal.