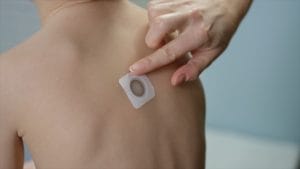
The manufacturer of the Viaskin Peanut patch has resubmitted its biologics license application (or BLA) to the FDA – in the hope of getting approval for the peanut allergy treatment.
DBV Technologies had voluntarily withdrawn its earlier submission in December, 2018 after the FDA told the pharmaceutical company that its application to approve the desensitizing peanut patch for children lacked certain detail.
In a release issued August 7, 2019, the company says: “The submission addresses the additional data needed on manufacturing procedures and quality controls, which were communicated by the FDA.”
DBV has previously told Allergic Living that the FDA had not raised concerns related to the safety or efficacy of Viaskin Peanut in the BLA.
DBV Technologies chief executive, Daniel Tassé, called the BLA submission “a milestone.” He thanked his team for their efforts to address the FDA’s needs, saying: “we are one step closer towards potentially bringing Viaskin Peanut to patients.”
The Viaskin Peanut patch is designed to desensitize allergic children aged 4 to 11 through a skin patch method (known as epicutaneous immunotherapy). Results of two controlled clinical trials were included in the submission. DBV is also investigating the potential of this form of Viaskin patch therapy for milk and egg allergies.
In September 2019, the FDA will first consider the BLA application for a different form of desensitization therapy. Aimmune Therapeutics is applying for approval of its AR101 oral immunotherapy for peanut allergy in children and teens aged 4 to 17. Aimmune completed two large controlled clinical trials of this desensitization method, which involves consuming tiny amounts of the peanut biologic product on a continuing basis.
In a recent news statement, its president and CEO also noted that the company also has released “data that showed patients who received daily treatment with AR101 beyond one year experienced clinically meaningful improvements in disease-specific quality of life and continued immunomodulation.”
These are the first two therapies to seek FDA approval for any food allergy.





