Dr. Brett King says early results for the trials of a new drug to treat eczema are very encouraging. Photo: Michael Marsland
Dr. Brett King says early results for the trials of a new drug to treat eczema are very encouraging. Photo: Michael Marsland An exciting new era in eczema treatment may be around the corner, according to a new study.
Dr. Brett King and his team at Yale School of Medicine recently revealed findings from their research into the use of a rheumatoid arthritis medication, known as Xeljanz, to treat moderate to severe eczema.
“The improvement in redness, swelling, etc., was dramatic over only weeks,” according to Dr. King, an assistant professor of dermatology at Yale.
The study involved six patients for whom standard therapies, such as oral and topical steroids, were ineffective. In some cases, improvements were noticeable after just days of treatment. All participants in the study reported significant relief from profound itching and thickening of the skin, with no side effects.
“I have spent most of my life trying to hide my skin, having to wear long sleeves even in the summer. I would scratch until my skin was bloody in my sleep,” says study participant Cindy McDowell. “Xeljanz has literally saved my life.”
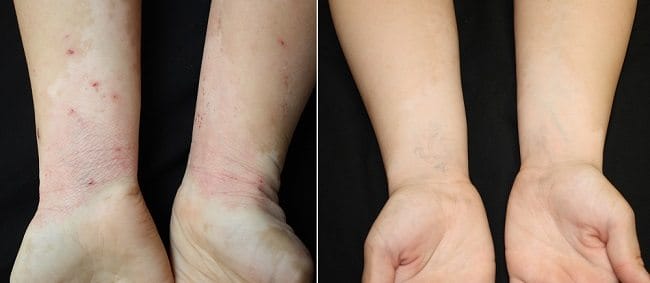 Forearms of eczema patient before and after treatment with arthritis medication. Photos: Dr. Brett King
Forearms of eczema patient before and after treatment with arthritis medication. Photos: Dr. Brett King Currently approved by the FDA for the treatment of rheumatoid arthritis, Xeljanz (tofacitinib citrate) is an immunomodulatory drug that works by altering the immune response.
Results from the study, published online in the Journal of the American Academy of Dermatology, come on the heels of another ground-breaking study confirming that eczema is an autoimmune disorder. In that study, patients received a weekly injection of dupilumab, a drug that reverses the immune response that causes the cracked, itchy skin of eczema. Results from this study were so impressive that the FDA designated dupilumab a “breakthrough therapy” to expedite its development.
Currently in clinical trials, topical tofacitinib has not yet been approved by the FDA. Oral tofacitinib has only been tested in adults. “If a topical formulation of tofacitinib becomes available in the future,” Dr. King says, “then physicians will be able to evaluate more safely its use in children.”