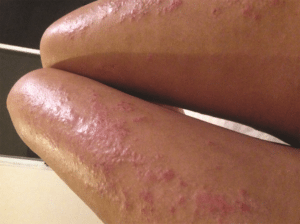
 The lesions on a woman's legs before dupilumab treatment. After treatment, her skin is clear. Photo: Emma Guttman-Yassky
The lesions on a woman's legs before dupilumab treatment. After treatment, her skin is clear. Photo: Emma Guttman-Yassky This is a sidebar to Allergic Living’s feature: Breakthrough Therapy Changing the Lives of Those Living With Eczema.
Dupilumab is a biologic therapy, which targets IL-4 and IL-13 proteins (called cytokines) that are drivers of inflammation in atopic dermatitis. But those same drivers are also implicated in other allergic diseases, and the pharmaceutical companies that launched the Dupixent brand in March, 2017 are investigating whether that biologic can be effective in asthma, nasal polyps and eosiniphilic esophagitis (EOE). As well, a food allergy study is in the works.
“You have to think about that our immune system is one system in the entire body,” explains Dr. Gianluca Pirozzi, Sanofi’s global R&D lead for dupilumab therapy. So if something goes wrong in the immune system, he says it will show up differently depending on factors such as genetic predisposition.
“In some patients it will show up more in the skin, in others it will show up in respiratory issues. In some other patients, it happens at the same time; they’ll have both,” he says.
Here is a summary of the status of other disease research with the dupilumab therapy.
Severe Asthma and Dupilumab Therapy
A Phase 2 clinical trial at 174 study sites showed favorable results with dupilumab therapy in adults with persistent asthma who weren’t getting symptom control with standard inhaled corticosteroids. Then in September 2017, topline results from a large Phase 3 study involving 1,900 patients at 413 global sites also met the primary objectives in 46 percent of patients (and higher in patient groups with eosinophilic cells).
Findings: In both trials, dupilumab therapy reduced asthma attacks and improved lung function, and the studies suggested it could be used alongside regular inhalers for control. The companies have submitted to the FDA for approval. There are several biologic competitors in the field of asthma, so dupilumab would face considerable competition. [Update: In late 2018, the FDA approved dupilumab for those over age 12 for corticosteroid-dependent asthma and EoE-based asthma.]
Nasal Polyps
This has reached the Phase 3 study stage as well, with participants getting shots for polyps at 13 sites in the U.S. and Europe. [Update: In February 2019, positive results were reported from two Phase 3 trials.]
Findings: In the Phase 2 study, significant improvement was seen in those with chronic sinusitis and polyps who got dupilumab shots in addition to a corticosteroid nasal spray. Also greatly improved was the sense of smell.
Food Allergies
They haven’t started enrolling yet, but Dr. Bola Akinlade, executive director at Regeneron Pharmaceuticals and the Dupixent program leader, says the companies plan on starting a dupilumab therapy and food allergies study “very soon.” [Update: A Phase 2 trial for food allergy is now underway.]
EOE
A study in adults is complete, but the results have not yet been released.
Dupilumab Therapy and Allergic Cross-over
As the lead on the nasal polyps study, Pirozzi met a patient who entered the trial when her third child was a year old. “She hadn’t had a sense of smell for years and years.” Once on the therapy she gave a powerful testimonial: “I can smell my baby for the first time,” she said. “I never smelled a baby in my life.”
Akinlade notes how often more than one of the diseases exists in the same person. Forty percent of participants in the clinical study with atopic dermatitis have asthma; 60 percent of the patients in the nasal polyps study have asthma, and 30 percent of those in the asthma study have nasal polyps.
“You can see there’s a lot of overlap,” he says. “Very often they have more than one disease.”





